No requirement
to label major
food allergens or
gluten-containing grains
in medication.
What is the ADINA Act?
H.R. 3821 – Formally known as the Allergen Disclosure In Non-food Articles Act, the Adina Act is a federal bill requiring plain-language labeling of all major food allergens and gluten-containing grains in both over-the-counter and prescription medications.
Why we need The ADINA Act
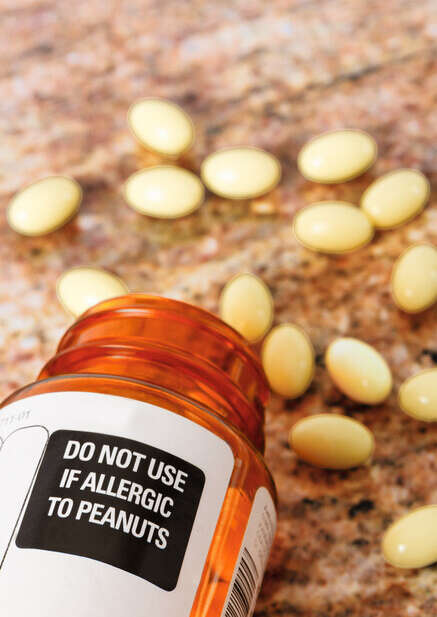
Transparency is overdue – Medications are ingested, inhaled, or applied to the body, and can cause the same serious or even life-threatening reactions as food… Yet in the U.S. there is no requirement to label these ingredients in medication.
Most oral medications contain known allergens – This is not a small problem. A 2019 study found that over 92% of oral medications contain at least one ingredient with documented case reports reflecting reactions in people with food allergies or sensitivities. These are most often found in the inactive ingredients, which on average make up 71% of a pill or capsule.
Even experts are left guessing – Pharmacists, physicians, and even manufacturer inserts often can’t confirm whether a medication contains a patient’s allergens or intolerances. Ingredient sourcing is unclear, and inserts are full of coded, clinical terms… with some components (like coatings, fillers, or manufacturing aids) often not being listed at all!
More than 100 million Americans would benefit – More than 100 million people live with conditions that require avoiding specific food ingredients… even in their medicine. This number includes 33 million individuals with food allergies, 3 million people with Celiac Disease, and another 66 million who struggle food intolerances. That is roughly 30% of the entire United States.
Smart regulation benefits everyone – Clear, consistent labeling reduces liability, improves consumer confidence, and streamlines healthcare decisions.
It’s just a label to some. But for millions with food allergies and gluten sensitivity, it’s everything.
The Facts
The critical information you need to know
- Allergens and gluten are most commonly found in inactive ingredients
On average, inactive ingredients make up 71% of a pill or capsule.
- There are over 1,000 inactive ingredients used in medications today.
At least 38 of those are known to cause allergic reactions, and nearly all pills contain at least one of them.
Pills contain an average of 8+ inactive ingredients, with some having up to 35!
Peanut oil, lactose, and certain sugars are common but not clearly labeled.
Even doctors and pharmacists can’t always verify what’s in a medication.
Source study: Reker et al., Science Translational Medicine (2019) / Video Credit: Diana Saville
Note: This third-party video was created before the ADINA Act. We did not contribute to its study or findings. Its inclusion is for educational purposes and does not imply endorsement, partnership, affiliation with the ADINA Act in any way.
Current co-sponsors of the ADINA Act
*As of October 1, 2025 in the 119th Congress.
A simple label could help millions
The ADINA Act asks are simple, common sense, and based in protecting those most vulnerable, with a need for clear and accessible information.
- No proprietary information needed
- We aren’t asking for medications to be reformulated
- It builds on existing labeling practices and leverages existing FDA structures
- The industry already knows what’s in it, it just needs to extend that disclosure to the label
